Description
Mutations in IDH1 and IDH2, the genes coding for isocitrate dehydrogenases 1 and 2, are common in several human cancers, such as leukemia and glioma, and result in overproduction of the (R)-enantiomer of 2- hydroxyglutarate [(R)-2-HG]. Elucidation of the role of IDH mutations and (R)-2-HG in leukemogenesis has been hampered by a lack of appropriate cell-based models. It has been recently reported that a canonical IDH1 mutant, IDH1 R132H, promoted cytokine independence and blocks differentiation in hematopoietic cells. These effects can be recapitulated by (R)-2-HG, but not (S)-2-HG, despite the fact that (S)-2-HG more potently inhibits enzymes previously linked to the pathogenesis of IDH mutant tumors, such as the 5'- methylcytosine hydroxylase TET2. This paradox is perhaps due to the ability of (S)-2-HG, but not (R)-2-HG, to inhibit the EglN prolyl hydroxylases. 2-HG has also been shown to inhibit the activity of multiple other a- KG-dependent dioxygenases, including the JmjC domain-containing histone demethylases (KDMs).
Product information
CAS Number: 103404-90-6
Molecular Weight: 192.08
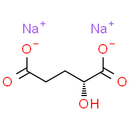
Formula: C5H6Na2O5
Synonym:
D-alpha-Hydroxyglutaric acid disodium
D-2-Hydroxypentanedioic acid disodium salt
Disodium (R)-2-Hydroxyglutarate
2R-hydroxy-pentanedioic acid, disodium salt
D-alpha-Hydroxyglutaric acid disodium salt
Pentanedioic acid, 2-hydroxy-, disodium salt, (R)-
D-2-Hydroxypentanedioic acid-2Na+
disodium (2R)-2-hydroxypentanedioate
r-2-hydroxyglutaric acid disodium salt
D-A-HYDROXYGLUTARIC ACID DISODIUM
MDK4906
[R, (+)]-2-Hydroxyglutaric acid disodium salt
Chemical Name: sodium (R)-2-hydroxypentanedioate
Smiles: [Na+].[Na+].[O-]C(=O)CC[C@@H](O)C([O-])=O
InChiKey: DZHFTEDSQFPDPP-HWYNEVGZSA-L
InChi: InChI=1S/C5H8O5.2Na/c6-3(5(9)10)1-2-4(7)8;;/h3,6H,1-2H2,(H,7,8)(H,9,10);;/q;2*+1/p-2/t3-;;/m1../s1
Technical Data
Appearance: Solid powder
Purity: ≥98% (or refer to the Certificate of Analysis)
Solubility: Water up to 100 mM
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical. This product is stable enough for a few weeks during ordinary shipping and time spent in Customs.
Storage Condition: Powder: 4oC for 1 year, -20oC for 3 years. DMSO: 4oC for 3 month.
Shelf Life: ≥36 months if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined.
HS Tariff Code: 382200
How to use
In Vitro:
(R)-2-HG was used at 100-250 μM final concentration in vitro and in cellular assays.
References:
- Losman JA, et al. (R)-2-Hydroxyglutarate Is Sufficient to Promote Leukemogenesis and Its Effects Are Reversible. (2013) Science. 339(6127):1621-5.
- Ye D, et al. R-2-Hydroxyglutarate as the Key Effector of IDH Mutations Promoting Oncogenesis. (2013) Cancer Cell. 23(3):274-6.
Products are for research use only. Not for human use.
Documents
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.