Description
CNV1014802 hydrochloride is a novel analgesic under development by Convergence Pharmaceuticals for the treatment of lumbosacral radiculopathy (sciatica) and trigeminal neuralgia (TGN). It acts as a selective, small-molecule, state-dependent Nav1.7 voltage-gated sodium channel blocker.
Product information
CAS Number: 934240-31-0
Molecular Weight: 350.82
Formula: C18H20ClFN2O2
Synonym:
GSK-1014802
CNV-1014802
Raxatrigine
Raxatrigine hydrochloride
Vixotrigine
GSK1014802
GSK 1014802
CNV1014802
CNV 1014802
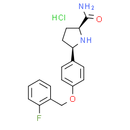
Chemical Name: (2S,5R)-5-(4-((2-fluorobenzyl)oxy)phenyl)pyrrolidine-2-carboxamide hydrochloride
Smiles: Cl.NC(=O)[C@@H]1CC[C@@H](N1)C1C=CC(=CC=1)OCC1=CC=CC=C1F
InChiKey: HEPUBAGOKRIZEO-PPPUBMIESA-N
InChi: InChI=1S/C18H19FN2O2.ClH/c19-15-4-2-1-3-13(15)11-23-14-7-5-12(6-8-14)16-9-10-17(21-16)18(20)22;/h1-8,16-17,21H,9-11H2,(H2,20,22);1H/t16-,17+;/m1./s1
Technical Data
Appearance: Solid Power
Purity: ≥98% (or refer to the Certificate of Analysis)
Solubility: Soluble in DMSO, not in water
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥12 months if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined.
HS Tariff Code: 382200
How to use
In Vitro:
Like lamotrigine, both GSK2 and GSK3 were able to prevent the deficit in reversal learning produced by PCP, thus confirming their potential in the treatment of cognitive symptoms of schizophrenia. However, higher doses than those required for anticonvulsant efficacy of the drugs were needed for activity in the reversal-learning model, suggesting a lower therapeutic window relative to mechanism-dependent central side effects for this indication. CNV1014802 hydrochloride (GSK-1014802) received orphan-drug designation from the US Food and Drug Administration in July 2013.
References:
- Deuis JR, Wingerd JS, Winter Z, Durek T, Dekan Z, Sousa SR, Zimmermann K, Hoffmann T, Weidner C, Nassar MA, Alewood PF, Lewis RJ, Vetter I. Analgesic Effects of GpTx-1, PF-04856264 and CNV1014802 in a Mouse Model of NaV1.7-Mediated Pain. Toxins (Basel). 2016 Mar 17;8(3). pii: E78. doi: 10.3390/toxins8030078. PubMed PMID: 26999206; PubMed Central PMCID: PMC4810223.
- Bagal SK, Chapman ML, Marron BE, Prime R, Storer RI, Swain NA. Recent progress in sodium channel modulators for pain. Bioorg Med Chem Lett. 2014 Aug 15;24(16):3690-9. doi: 10.1016/j.bmcl.2014.06.038. Epub 2014 Jun 21. Review. PubMed PMID: 25060923.
- Zakrzewska JM, Palmer J, Ettlin DA, Obermann M, Giblin GM, Morisset V, Tate S, Gunn K. Novel design for a phase IIa placebo-controlled, double-blind randomized withdrawal study to evaluate the safety and efficacy of CNV1014802 in patients with trigeminal neuralgia. Trials. 2013 Nov 23;14:402. doi: 10.1186/1745-6215-14-402. PubMed PMID: 24267010; PubMed Central PMCID: PMC4222641.
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.