Description
Amphotericin B trihydrate, a polyene antibiotic, is first isolated from fermenter cultures of Streptomyces nodosus. Amphotericin B trihydrate also possesses antileishmanial activity.
Product information
CAS Number: 1202017-46-6
Molecular Weight: 978.12
Formula: C47H79NO20
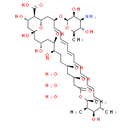
Chemical Name: (1R, 3S, 5R, 6R, 9R, 11R, 15S, 16R, 17R, 18S, 19E, 21E, 23E, 25E, 27E, 29E, 31E, 33R, 35S, 36R, 37S)-33-(((2R, 3S, 4S, 5S, 6R)-4-amino-3, 5-dihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-1, 3, 5, 6, 9, 11, 17, 37-octahydroxy-15, 16, 18-trimethyl-13-oxo-14, 39-dioxabicyclo[33.3.1]nonatriaconta-19, 21, 23, 25, 27, 29, 31-heptaene-36-carboxylic acid trihydrate
Smiles: O.O.O.C[C@H]1C=CC=CC=CC=CC=CC=CC=C[C@@H](C[C@@H]2O[C@@](O)(C[C@H](O)[C@H]2C(O)=O)C[C@@H](O)C[C@@H](O)[C@H](O)CC[C@@H](O)C[C@@H](O)CC(=O)O[C@@H](C)[C@H](C)[C@@H]1O)O[C@@H]1O[C@H](C)[C@@H](O)[C@H](N)[C@@H]1O |t:2,4,6,8,10,12,14|
InChiKey: QEWGLCMWTBNETQ-UBYVJTKBSA-N
InChi: InChI=1S/C47H73NO17.3H2O/c1-27-17-15-13-11-9-7-5-6-8-10-12-14-16-18-34(64-46-44(58)41(48)43(57)30(4)63-46)24-38-40(45(59)60)37(54)26-47(61,65-38)25-33(51)22-36(53)35(52)20-19-31(49)21-32(50)23-39(55)62-29(3)28(2)42(27)56;;;/h5-18,27-38,40-44,46,49-54,56-58,61H,19-26,48H2,1-4H3,(H,59,60);3*1H2/b6-5-,9-7-,10-8-,13-11-,14-12-,17-15-,18-16-;;;/t27-,28-,29-,30+,31+,32+,33-,34-,35+,36+,37-,38-,40+,41-,42+,43+,44-,46-,47+;;;/m0.../s1
Technical Data
Appearance: Solid Power
Purity: ≥98% (or refer to the Certificate of Analysis)
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥12 months if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined
HS Tariff Code: 382200
How to use
In Vitro:
Amphotericin B interacts with cholesterol, the major sterol of mammal membranes, thus limiting the usefulness of Amphotericin B due to its relatively high toxicity. Amphotericin B is dispersed as a pre-micellar or as a highly aggregated state in the subphase. Amphotericin B only kills unicellular Leishmania promastigotes (LPs) when aqueous pores permeable to small cations and anions are formed. Amphotericin B (0.1 mM) induces a polarization potential, indicating K+ leakage in KCl-loaded liposomes suspended in an iso-osmotic sucrose solution. Amphotericin B (0.05 mM) exhibits a nearly total collapse of the negative membrane potential, indicating Na+ entry into the cells.
In Vivo:
Amphotericin B results in prolonging the incubation time and decreasing PrPSc accumulation in the hamster scrapie model. Amphotericin B markedly reduces PrPSc levels in mice with transmissible subacute spongiform encephalopathies (TSSE). Amphotericin B exerts a direct effect on Plasmodium falciparum and influences eryptosis of infected erythrocytes, parasitemia and hostsurvival in murine malaria. Amphotericin B tends to delay the increase of parasitemia and significantly delays host death plasmodium berghei-infected mice.
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.