Description
Camptothecin (CPT) is a cytotoxic quinoline alkaloid which inhibits the DNA enzyme topoisomerase I (topo I). It was discovered in 1966 by M. E. Wall and M. C. Wani in systematic screening of natural products for anticancer drugs. It was isolated from the bark and stem of Camptotheca acuminata (Camptotheca, Happy tree), a tree native to China used as a cancer treatment in Traditional Chinese Medicine. CPT showed remarkable anticancer activity in preliminary clinical trials but also low solubility and (high) adverse drug reaction. Because of these disadvantages synthetic and medicinal chemists have developed numerous syntheses of Camptothecin and various derivatives to increase the benefits of the chemical, with good results. Two CPT analogues have been approved and are used in cancer chemotherapy today, topotecan and irinotecan.
Product information
CAS Number: 7689-03-4
Molecular Weight: 348.35
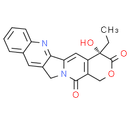
Formula: C20H16N2O4
Synonym:
(S)-(+)-Camptothecin
d-Camptothecin
20(S)-Camptothecine
(+)-Camptothecin
Camptothecin
CPT
Chemical Name: (S)-4-ethyl-4-hydroxy-1H-pyrano[3', 4':6, 7]indolizino[1, 2-b]quinoline-3, 14(4H, 12H)-dione
Smiles: CC[C@]1(O)C2C=C3C4=NC5=CC=CC=C5C=C4CN3C(=O)C=2COC1=O
InChiKey: VSJKWCGYPAHWDS-FQEVSTJZSA-N
InChi: InChI=1S/C20H16N2O4/c1-2-20(25)14-8-16-17-12(7-11-5-3-4-6-15(11)21-17)9-22(16)18(23)13(14)10-26-19(20)24/h3-8,25H,2,9-10H2,1H3/t20-/m0/s1
Technical Data
Appearance: Solid Power
Purity: ≥98% (or refer to the Certificate of Analysis)
Solubility: DMSO: 3 mg/mL(8.61 mM).
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥12 months if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined
HS Tariff Code: 382200
How to use
In Vitro:
Camptothecin, a plant alkaloid orignially isolated from Camptotheca acuminate in 1966. Camptothecin is noted to halt cells during the S phase of mitosis. Camptothecin displays nanomolar potency in cytotoxicity against many human tumor cell lines, including HT29, LOX, SKOV3, and SKVLB, with IC50 values ranging from 37 nM to 48 nM. In combination with TNF, Camptothecin induces apoptosis in primary mouse hepatocytes, with an IC50 value of 13 μM. Camptothecin also abrogated the TNF-induced NF-κB Activation, as well as the expression of TNF-receptor associated factor 2 (TRAF2), X-linked inhibitor of apoptosis protein (X-IAP), and FLICE-inhibitory protein (FLIP). In HCT116 cells, Camptothecin (5 μM) induces proteasome-mediated degradation of mixed lineage leukemia 5 (MLL5) protein, which leads to phosphorylation of p53 at Ser392. Due to the low solubility and adverse effects of Camptothecin, various Camptothecin analogues have been developed, and two of them, topotecan and irinotecan, has been approved by FDA and are used in cancer chemotherapy.
In Vivo:
Camptothecin (8 mg/kg) displays complete growth inhibition and regression in mice xenografts of various tumors, including colon, lung, breast, stomach, and ovary tumors. In mice, combinations of Camptothecin (50 mg/kg) and TNF (5 and 7 μg/kg), but not Camptothecin alone, induces liver damage.
References:
- Giovanella BC, et al. Cancer Res, 1991, 51(11), 3052-3055.
- Luzzio MJ, et al. J Med Chem, 1995, 38(3), 395-401.
- Wall ME, et al. J Am Chem Soc, 1966, 88 (16), 3888–3890.
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.