Description
Bilastine (trade name Bilaxten) is a second generation antihistamine drug for the treatment of allergic rhinoconjunctivitis and urticaria (hives). It exerts its effect as a selective histamine H1 receptor antagonist, and has a potency similar to cetirizine and is superior to fexofenadine. It was developed in Spain by FAES Farma. Bilastine is approved in the European Union for the symptomatic treatment of allergic rhinoconjunctivitis and urticaria, but it is not approved by the U.S. Food and Drug Administration for any use in the United States. Bilastine has been effective in the treatment of ocular symptoms and diseases of allergies, including rhinoconjuctivitis. Additionally, bilastine has been shown to improve quality of life, and all nasal and ocular symptoms related to allergic rhinitis.
Product information
CAS Number: 202189-78-4
Molecular Weight: 463.61
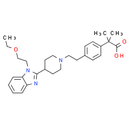
Formula: C28H37N3O3
Synonym:
Ilaxten
Chemical Name: 2-[4-(2-{4-[1-(2-ethoxyethyl)-1H-1,3-benzodiazol-2-yl]piperidin-1-yl}ethyl)phenyl]-2-methylpropanoic acid
Smiles: CCOCCN1C(=NC2=CC=CC=C12)C1CCN(CCC2C=CC(=CC=2)C(C)(C)C(O)=O)CC1
InChiKey: ACCMWZWAEFYUGZ-UHFFFAOYSA-N
InChi: InChI=1S/C28H37N3O3/c1-4-34-20-19-31-25-8-6-5-7-24(25)29-26(31)22-14-17-30(18-15-22)16-13-21-9-11-23(12-10-21)28(2,3)27(32)33/h5-12,22H,4,13-20H2,1-3H3,(H,32,33)
Technical Data
Appearance: Solid Power
Purity: ≥98% (or refer to the Certificate of Analysis)
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥12 months if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined
HS Tariff Code: 382200
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.