Description
Rilpivirine is a pharmaceutical drug, developed by Tibotec, for the treatment of HIV infection. It is a second-generation non-nucleoside reverse transcriptase inhibitor (NNRTI) with higher potency, longer half-life and reduced side-effect profile compared with older NNRTIs, such as efavirenz. Rilpivirine entered phase III clinical trials in April 2008, and was approved for use in the United States in May 2011. A fixed-dose drug combining rilpivirine with emtricitabine and tenofovir, was approved by the U.S. Food and Drug Administration in August 2011 under the brand name Complera.
Product information
CAS Number: 500287-72-9
Molecular Weight: 366.42
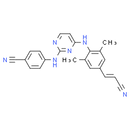
Formula: C22H18N6
Chemical Name: 4-{[4-({4-[(1E)-2-cyanoeth-1-en-1-yl]-2,6-dimethylphenyl}amino)pyrimidin-2-yl]amino}benzonitrile
Smiles: CC1C=C(C=C(C)C=1NC1=CC=NC(NC2C=CC(=CC=2)C#N)=N1)/C=C/C#N
InChiKey: YIBOMRUWOWDFLG-ONEGZZNKSA-N
InChi: InChI=1S/C22H18N6/c1-15-12-18(4-3-10-23)13-16(2)21(15)27-20-9-11-25-22(28-20)26-19-7-5-17(14-24)6-8-19/h3-9,11-13H,1-2H3,(H2,25,26,27,28)/b4-3+
Technical Data
Appearance: Solid Power
Purity: ≥98% (or refer to the Certificate of Analysis)
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥360 days if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined
HS Tariff Code: 382200
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.