Description
Sotagliflozin, also known as LX4211, is an orally active and a dual SGLT1/SGLT2 inhibitor, which improves glycemic control in patients with type 2 diabetes in a randomized, placebo-controlled trial. LX4211 increases serum glucagon-like peptide 1 and peptide YY levels by reducing sodium/glucose cotransporter 1 (SGLT1)-mediated absorption of intestinal glucose. Sotagliflozin is currently being developed by Lexicon for the treatment of type 1 and type 2 diabetes mellitus. Sotagliflozin may be an effective and promising medication for treating not only Type 2 diabetes (the common target for non-insulin medications for diabetes), but also Type 1 as well.
Product information
CAS Number: 1018899-04-1
Molecular Weight: 424.94
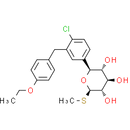
Formula: C21H25ClO5S
Chemical Name: (2S,3R,4R,5S,6R)-2-{4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl}-6-(methylsulfanyl)oxane-3,4,5-triol
Smiles: CCOC1C=CC(CC2=CC(=CC=C2Cl)[C@@H]2O[C@H](SC)[C@@H](O)[C@H](O)[C@H]2O)=CC=1
InChiKey: QKDRXGFQVGOQKS-CRSSMBPESA-N
InChi: InChI=1S/C21H25ClO5S/c1-3-26-15-7-4-12(5-8-15)10-14-11-13(6-9-16(14)22)20-18(24)17(23)19(25)21(27-20)28-2/h4-9,11,17-21,23-25H,3,10H2,1-2H3/t17-,18-,19+,20+,21-/m1/s1
Technical Data
Appearance: Solid Power
Purity: ≥98% (or refer to the Certificate of Analysis)
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥360 days if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined
HS Tariff Code: 382200
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.