Description
Pasireotide, also known as SOM230, is an orphan drug approved for the treatment of Cushing's disease in patients who fail or are ineligible for surgical therapy. It was developed by Novartis. Pasireotide is a somatostatin analog with a 40-fold increased affinity to somatostatin receptor 5 compared to other somatostatin analogs.
Product information
CAS Number: 396091-73-9
Molecular Weight: 1047.21
Formula: C58H66N10O9
Synonym:
SOM 230
Signifor
SOM 320
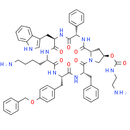
Chemical Name: (3S,6R,9S,12S,15S,19R,20aS)-9-(4-aminobutyl)-15-benzyl-12-{[4-(benzyloxy)phenyl]methyl}-6-[(1H-indol-3-yl)methyl]-1,4,7,10,13,16-hexaoxo-3-phenyl-icosahydropyrrolo[1,2-a]1,4,7,10,13,16-hexaazacyclooctadecan-19-yl N-(2-aminoethyl)carbamate
Smiles: NCCCC[C@@H]1NC(=O)[C@@H](CC2=CNC3=CC=CC=C23)NC(=O)[C@@H](NC(=O)[C@@H]2C[C@H](CN2C(=O)[C@H](CC2=CC=CC=C2)NC(=O)[C@H](CC2=CC=C(C=C2)OCC2=CC=CC=C2)NC1=O)OC(=O)NCCN)C1=CC=CC=C1
InChiKey: VMZMNAABQBOLAK-DBILLSOUSA-N
InChi: InChI=1S/C58H66N10O9/c59-27-13-12-22-46-52(69)64-47(30-38-23-25-42(26-24-38)76-36-39-16-6-2-7-17-39)53(70)66-49(31-37-14-4-1-5-15-37)57(74)68-35-43(77-58(75)61-29-28-60)33-50(68)55(72)67-51(40-18-8-3-9-19-40)56(73)65-48(54(71)63-46)32-41-34-62-45-21-11-10-20-44(41)45/h1-11,14-21,23-26,34,43,46-51,62H,12-13,22,27-33,35-36,59-60H2,(H,61,75)(H,63,71)(H,64,69)(H,65,73)(H,66,70)(H,67,72)/t43-,46+,47+,48-,49+,50+,51+/m1/s1
Technical Data
Appearance: Solid Power
Purity: ≥98% (or refer to the Certificate of Analysis)
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥12 months if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined
HS Tariff Code: 382200
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.