Description
Linagliptin, also known as BI-1356, is a DPP-4 inhibitor developed by Boehringer Ingelheim for treatment of type II diabetes. Linagliptin (once-daily) was approved by the US FDA on 2 May 2011 for treatment of type II diabetes. It is being marketed by Boehringer Ingelheim and Lilly.
Product information
CAS Number: 668270-12-0
Molecular Weight: 472.54
Formula: C25H28N8O2
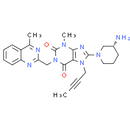
Chemical Name: 8-[(3R)-3-aminopiperidin-1-yl]-7-(but-2-yn-1-yl)-3-methyl-1-[(4-methylquinazolin-2-yl)methyl]-2,3,6,7-tetrahydro-1H-purine-2,6-dione
Smiles: CN1C2N=C(N(CC#CC)C=2C(=O)N(CC2=NC3=CC=CC=C3C(C)=N2)C1=O)N1C[C@H](N)CCC1
InChiKey: LTXREWYXXSTFRX-QGZVFWFLSA-N
InChi: InChI=1S/C25H28N8O2/c1-4-5-13-32-21-22(29-24(32)31-12-8-9-17(26)14-31)30(3)25(35)33(23(21)34)15-20-27-16(2)18-10-6-7-11-19(18)28-20/h6-7,10-11,17H,8-9,12-15,26H2,1-3H3/t17-/m1/s1
Technical Data
Appearance: Solid Power
Purity: ≥98% (or refer to the Certificate of Analysis)
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥360 days if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined
HS Tariff Code: 382200
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.