Description
Sitravatinib malate (MGCD516 malate) is an orally bioavailable receptor tyrosine kinase (RTK) inhibitor with IC50s of 1.5 nM, 2 nM, 2 nM, 5 nM, 6 nM, 6 nM, 8 nM, 0.5 nM, 29 nM, 5 nM, and 9 nM for Axl, MER, VEGFR3, VEGFR2, VEGFR1, KIT, FLT3, DDR2, DDR1, TRKA, TRKB, respectively. Sitravatinib malate shows potent single-agent antitumor efficacy and enhances the activity of PD-1 blockade through promoting an antitumor immune microenvironment.
Product information
CAS Number: 2244864-88-6
Molecular Weight: 763.76
Formula: C37H35F2N5O9S
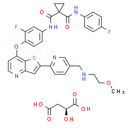
Chemical Name: (2S)-2-hydroxybutanedioic acid; N'1-(3-fluoro-4-{[2-(5-{[(2-methoxyethyl)amino]methyl}pyridin-2-yl)thieno[3,2-b]pyridin-7-yl]oxy}phenyl)-N1-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide
Smiles: COCCNCC1C=CC(=NC=1)C1=CC2N=CC=C(OC3=CC=C(C=C3F)NC(=O)C3(CC3)C(=O)NC3C=CC(F)=CC=3)C=2S1.O[C@@H](CC(O)=O)C(O)=O
InChiKey: GDLGZWLGDROYHH-WNQIDUERSA-N
InChi: InChI=1S/C33H29F2N5O4S.C4H6O5/c1-43-15-14-36-18-20-2-8-25(38-19-20)29-17-26-30(45-29)28(10-13-37-26)44-27-9-7-23(16-24(27)35)40-32(42)33(11-12-33)31(41)39-22-5-3-21(34)4-6-22;5-2(4(8)9)1-3(6)7/h2-10,13,16-17,19,36H,11-12,14-15,18H2,1H3,(H,39,41)(H,40,42);2,5H,1H2,(H,6,7)(H,8,9)/t;2-/m.0/s1
Technical Data
Appearance: Solid Power
Purity: ≥98% (or refer to the Certificate of Analysis)
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥12 months if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined
HS Tariff Code: 382200
How to use
In Vitro:
Sitravatinib (0.01 nM-10 μM; 14 days) reduces colony formation in a dose-dependent manner in KLN205 and E0771 cell lines. Sitravatinib (0.001-10 μM; 5 days) inhibits tumor cell viability with IC50s of approximately 1 μM in KLN205, E0771 and CT1B-A5 cell lines.
In Vivo:
Sitravatinib (20 mg/kg; p.o.; once per day for 6 days) significantly inhibits tumor progression and induces tumor regression in C57BL/6 mice bearing CT1B-A5 cells model.
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.