Description
Chebulinic Acid is a potent protectant against glutamate-induced neuronal cell death. It has been shown to significantly reduce intracellular reactive oxygen species (ROS) production and Ca2+ influx induced by glutamate, decrease the phosphorylation of mitogen-activated protein kinases (MAPKs), including ERK1/2, JNK, and p38, as well as inhibit pro-apoptotic Bax and increase anti-apoptotic Bcl-2 protein expression.
Product information
CAS Number: 18942-26-2
Molecular Weight: 956.68
Formula: C41H32O27
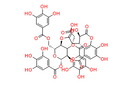
Chemical Name: 2-((3S,3aS,4S,7R,8R,10S,11R,17S)-3,15,16-Trihydroxy-2,5,13-trioxo-10,17-bis((3,4,5-trihydroxybenzoyl)oxy)-8-(((3,4,5-trihydroxybenzoyl)oxy)methyl)-2,3,3a,4,5,7,8,10,11,13-decahydro-7,11-methano[1,4,7]trioxacyclotridecino[11,10,9-de]chromen-4-yl)acetic acid
Smiles: O[C@@H]1[C@H]2[C@H](CC(O)=O)C(=O)O[C@@H]3[C@@H](COC(=O)C4=CC(O)=C(O)C(O)=C4)O[C@@H](OC(=O)C4=CC(O)=C(O)C(O)=C4)[C@H](OC(=O)C4=CC(O)=C(O)C(OC1=O)=C42)C3OC(=O)C1=CC(O)=C(O)C(O)=C1
InChiKey: YGVHOSGNOYKRIH-REKMSKHASA-N
InChi: InChI=1S/C41H32O27/c42-15-1-10(2-16(43)26(15)51)35(56)62-9-22-31-33(66-36(57)11-3-17(44)27(52)18(45)4-11)34(41(63-22)68-37(58)12-5-19(46)28(53)20(47)6-12)67-38(59)13-7-21(48)29(54)32-25(13)24(30(55)40(61)65-32)14(8-23(49)50)39(60)64-31/h1-7,14,22,24,30-31,33-34,41-48,51-55H,8-9H2,(H,49,50)/t14-,22+,24-,30+,31+,33?,34+,41-/m0/s1
Technical Data
Appearance: Solid Power.
Purity: ≥98% (or refer to the Certificate of Analysis)
Solubility: Soluble in DMSO
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥12 months if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined.
HS Tariff Code: 382200
How to use
In Vitro:
In vitro: binding of Chebulinic acid causes displacement of catalytic Tyr129 away from its target DNA-phosphate molecule. Chebulinic acid reduce the expression and activity of MMP-2 at an ED50 value of 100 μM. EMT (Epithelial to Mesenchymal Transition) is found to be induced in ARPE-19 cells, through SMAD-3 phosphorylation and it is inhibited by CA. chebulinic acid significantly inhibited H+ K+-ATPase activity in vitrowith IC50 of 65.01 μg/ml.
References:
- Song JH, Shin MS, Hwang GS, Oh ST, Hwang JJ, Kang KS. Chebulinic acid attenuates glutamate-induced HT22 cell death by inhibiting oxidative stress, calcium influx and MAPKs phosphorylation. Bioorg Med Chem Lett. 2017 Dec 29. pii: S0960-894X(17)31236-2. doi: 10.1016/j.bmcl.2017.12.062. [Epub ahead of print] PubMed PMID: 29317168.
- Lopez HL, Habowski SM, Sandrock JE, Raub B, Kedia A, Bruno EJ, Ziegenfuss TN. Effects of dietary supplementation with a standardized aqueous extract of Terminalia chebula fruit (AyuFlex(®)) on joint mobility, comfort, and functional capacity in healthy overweight subjects: a randomized placebo-controlled clinical trial. BMC Complement Altern Med. 2017 Oct 2;17(1):475. doi: 10.1186/s12906-017-1977-8. PubMed PMID: 28969626; PubMed Central PMCID: PMC5625793.
- Song IS, Jeong YJ, Park JH, Shim S, Jang SW. Chebulinic acid inhibits smooth muscle cell migration by suppressing PDGF-Rβ phosphorylation and inhibiting matrix metalloproteinase-2 expression. Sci Rep. 2017 Sep 18;7(1):11797. doi: 10.1038/s41598-017-12221-w. PubMed PMID: 28924208; PubMed Central PMCID: PMC5603554.
- Chhabra S, Mishra T, Kumar Y, Thacker G, Kanojiya S, Chattopadhyay N, Narender T, Trivedi AK. Chebulinic Acid Isolated From the Fruits of Terminalia chebula Specifically Induces Apoptosis in Acute Myeloid Leukemia Cells. Phytother Res. 2017 Dec;31(12):1849-1857. doi: 10.1002/ptr.5927. Epub 2017 Sep 18. PubMed PMID: 28921713.
- Vu TT, Kim H, Tran VK, Vu HD, Hoang TX, Han JW, Choi YH, Jang KS, Choi GJ, Kim JC. Antibacterial activity of tannins isolated from Sapium baccatum extract and use for control of tomato bacterial wilt. PLoS One. 2017 Jul 25;12(7):e0181499. doi: 10.1371/journal.pone.0181499. eCollection 2017. PubMed PMID: 28742863; PubMed Central PMCID: PMC5526539.
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.